
CosmedGroup
Ensuring Food Safety and Purity with Advanced Pasteurization and Sterilization Solutions
Cosmed Group provides pasteurization and sterilization services and technologies to food producers and manufacturers through a network of contract processing facilities. The company offers several processes, including their patented steam pasteurization H20 Express™ technology, to ensure the safety and purity of herbs, spices, nuts, fruits, vegetables, birdseed, nutraceuticals, grains, and other agricultural commodities. Additionally, Cosmed Group provides equipment and consulting services to support customers operating pasteurization and sterilization processes in-house.
What you should know

Blog
Biological Indicators / Parametric Release
Biological indicators (BIs) are essential tools for monitoring the efficacy of terminal sterilization processes. These indicators contain highly resistant microorganisms that are intended to be more difficult to kill.
September 05, 2024
Blog
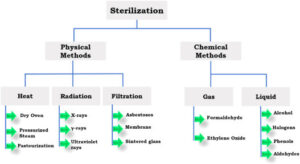
Which Method of Sterilization Should I Use?
That is the big question. Fortunately, when you boil it down to its essence, it’s actually quite easy to answer. There are only one or two questions that need to be asked and answered, and then the decision should be straightforward.
June 04, 2024

Blog
Saving Time using EO
The goal of all sterilants is to either kill microorganisms or make them incapable of replicating.
February 29, 2024

environmental control systems
A New Day for a Medical Device Manufacturer
Cosmed Group, Inc. (Cosmed) is pleased to announce the completion of an agreement to supply ethylene oxide sterilization chambers, Glygen™ environmental control systems, and facility design services to a medical device manufacturer.

