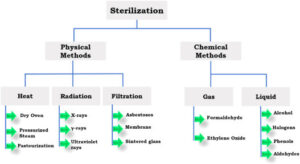
The breakdown of industry sterilization is generally agreed to be roughly 52% ethylene oxide (EO) gas, 36% Gamma radiation, and 7% E-beam radiation. The remaining 5% are various modalities like steam, hydrogen peroxide, gas plasma, nitrogen dioxide (NO2), peracetic acid (PAA) and some others. In total, there are only about 25 sterilization methods known to mankind.
Gaseous sterilization
EO is the most popular and growing slightly because it is the most material friendly. As devices get more complex (e.g. combination products, custom kits, others), they may be made of multiple materials, some of which may not be compatible with radiation. EO can also be used in some instances where products contain computer chips as they will not affect their performance, whereas radiation processing will generally not work for these items. One of its main historical drawbacks is that it typically had the longest turnaround time as compared to radiation processing. However, with the advent of things like combining All-In-One processing with parametric release, the turnaround time, where this can be used, rivals that of the radiation techniques.
Moist heat (steam) sterilization is arguably the best method of sterilization since it is the least expensive, requires the lowest capital investment for the equipment, non-toxic to workers, leaves no residue, and the fastest method. However, due to the high temperatures required, many materials cannot tolerate this technique so these products have to use some other sterilization method.
Peracetic Acid (PAA) can be done at room temperature, which has some advantages for products that are temperature sensitive. In is a vapor formed by a reaction of acetic acid and hydrogen peroxide, with the addition of a catalyst. The compounds exist in equilibrium and once the compound decomposes, the byproducts are oxygen, carbon dioxide, and water. During sterilization the vapor interacts with bacteria’s cellular constituents, breaking them down and inactivating routine functionality. The vapor process is effective against all types of microorganisms.
Radiation sterilization
In the radiation arena you have three options, Gamma, E-Beam, and X-ray. All of these methods can be performed with relatively quick turnaround. One of the biggest factors to be addressed with this modality is material compatibility. Some materials can discolor, cross link, become brittle, increase durometer, etc.
Gamma processing has great penetration and most types of product that are radiation compatible can be effectively processed using this method. The typical turnaround time for gamma processing may be a bit longer than the other two radiation methods since the sterilization facility typically waits for other products with similar dose ranges so they can all be processed at the same time. There is some concern in the marketplace about the diminishing supply and resultant rising cost of the cobalt used as the radioactive source for this methodology. In some instances, this may be causing companies to consider alternate radiation technologies, or perhaps gaseous sterilization technologies.
E-beam, although a smaller overall market share than gamma, is the fastest growing of the radiation processing methodologies on a percentage basis. It has some advantages because it’s source of energy is electricity and, in contrast to gamma, the radiation source can be turned off when it is not being used. E-beam has the quickest turnaround time of all of the radiation methodologies as it can process each product in seconds and therefore a load of product in a short time. Because of the short exposure time, this technology may provide less radiation degradation to the materials being processed. In addition, dose rates can be rapidly adjusted for varying products, which also helps provide a quicker turnaround time. The limitation with this process is the density of the load as some types of products may not lend themselves to E-beam processing.
Among the three radiation processes, X-ray is one that may be the best from several aspects. It has a slightly greater penetration potential than Gamma and, along with that, the variability in dose range across the load is generally lower. Because of the superior penetration potential, processing may often be performed by the pallet, which saves time and labor costs. For niche market temperature sensitive products, X-ray also typically has the lowest heat production, which gives it the edge for those types of products. Despite the advantages of X-ray over the other radiation methodologies, the number one drawback at this time is the economics as this method is more costly than the other two methodologies. As evidence of this, there is currently only one contract sterilizer doing this type of processing for medical devices, with a second one coming on-line sometime this calendar year.
Going in house
There is an increasing trend for some companies to take sterilization in-house, both in OEM and contract manufacturing. The biggest decision point is the overall economics and how big of a role sterilization plays in the final cost of the product. Other important factors that play into the decision to move this process in-house include such things as quality, turnaround time, lower inventory, customer service, etc. It is important to note that several other factors need to be carefully assessed before making the move. Some factors to consider include safety, building space for the process, in-house expertise, and liability, just to name a few.
With this in mind, the easiest of the primary methods (not including steam sterilization, which is and always has been primarily in-house) to bring in house is EO, with the next easiest method being E-beam. While it would be possible for a company to bring in gamma or X-ray processing, it would be uncommon for a company to do so based on the large capital investment cost. According to some manufactures, what makes sense is to do the sterilization in-house when that is the best choice, and use contract sterilization where it has distinct advantages.
Which Method of Sterilization Should I Use?
That is the big question. Fortunately, when you boil it down to its essence, it’s actually quite easy to answer. There are only one or two questions that need to be asked and answered, and then the decision should be straightforward.
What method(s) will work to sterilize my product in a safe and effective manner? Often the answer to that question dictates the method as one may only be able to use EO (or another gaseous niche method), or radiation may be the only method that works. If the product is designed with sterilization in mind and can be processed with either a gaseous or radiation methods, then the second question comes into play.
What methods will sterilize my product in the most cost effective manner? This is a bit more complex question as it needs to take into account things like product and packaging materials and design, cost of the sterilization equipment and upkeep of that equipment, product transportation, sterilization indicator system, validation and requalification costs, routine sterilization costs, turnaround time, etc.
Once these questions are properly and thoughtfully answered, the decision as to the sterilization methodology, and whether to do this processing at a contract sterilization organization or in-house, should become self-evident.