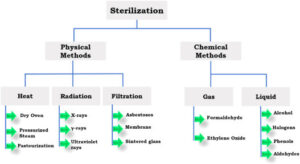
Statement: Biological indicators (BIs) are essential tools for monitoring the efficacy of terminal sterilization processes. These indicators contain highly resistant microorganisms that are intended to be more difficult to kill than the typical microorganisms found in the product being sterilized. By subjecting these indicators to the sterilization process, the effectiveness of the sterilization cycle can be verified.
Response: It is important to note that biological indicators by themselves do not prove that a product is sterile to the required sterility assurance level. In order to accomplish that you must have three things. Number one is a properly conducted and acceptable process validation. Second is that the process parameters must be within specification. And finally, the sterility released mechanism, whether biological indicators or parametric release (if allowed for the method of sterilization in question), must be acceptable. Please note that if parametric release is allowed, that it alone can provide the required verification that the process has been carried out successfully and that biological indicators would not be required.