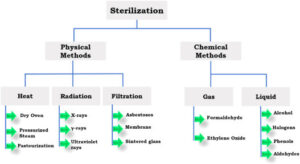
The goal of all sterilants is to either kill microorganisms or make them incapable of replicating.
The goal of all sterilants is to either kill microorganisms or make them incapable of replicating. “You can have one bad germ, but if it can’t reproduce itself, it means nothing,” Opie adds.
Typically, medical device manufacturers design their methods to achieve a sterility assurance level (SAL) of 10-6. At that level, the chances are one in a million that one microorganism remains viable, explains Smith, who has more than 30 years of experience in the field of radiation.
To reach such a SAL, different sterilization methods take different approaches.
Conventional EO sterilization, for example, historically relied on a three-phase process that begins with preconditioning. Pallets of medical devices are placed in a room, chamber or cell, where they are exposed to heat and humidity for a defined period to acclimate the devices to the sterilization conditions and to make the microorganisms more susceptible to the sterilization process.
In the second phase, the pallets are placed in a sterilizer, which can range from the size of a tabletop to a full tractor trailer. The medical devices are sterilized in their final packaging, typically corrugated cardboard boxes.
In the United States, most EO sterilizers use 100 percent EO, as opposed to a blend of EO and carbon dioxide, Houghtling notes. When 100 percent EO is used, the process is done under vacuum, creating an environment that is lower than atmospheric pressure. After air is removed from the sterilizing chamber and humidity added, EO is introduced. Several hours are required for EO to permeate throughout the load and kill microorganisms to the designated SAL. EO is then flushed out of the chamber.
For EO sterilization, it’s essential that devices are packaged in gas-permeable sterile barriers. The pores in the barriers are large enough to allow EO to flow in and out but too small to allow microorganisms in. As a consequence, once EO kills the existing microorganisms on the devices, the devices remain sterile until their gas-permeable barriers are opened.
The last phase of EO sterilization is aeration to further remove EO. This is done in a room, chamber or cell with heat, but without humidity. Aeration reduces EO concentrations to or below permissible levels for the safety of both the workers who handle the devices and the patients who are treated with them.
Coming more into vogue in EO sterilization is all-in-one processing, in which all three phases take place in the sterilizer instead of in three different areas. The all-in-one process is safer for workers because they do not move the pallets until the product is nearly fully aerated. It also reduces process deviations and product damage.
On the other hand, because the entire process is executed in a sterilizer, the amount of time that the devices are in the sterilizer roughly doubles, cutting throughput in half. So to process the same volume, all-in-one processing requires roughly doubling the number or size of the sterilizers. In addition, sterilizers are much more expensive than preconditioning and aeration areas.
However, the time required for EO sterilization can be reduced significantly by combining all-in-one processing with dynamic environmental conditioning (DEC), parametric release and in-chamber aeration, Houghtling notes.
DEC, which works best for products that can withstand deep vacuums, can be used to more quickly and evenly heat and humidify product loads.
Parametric release eliminates the need for using biological indicators to monitor whether the designated SAL was reached, which typically takes two to seven days. In addition, the dynamics of the sterilization chamber can be used to speed up aeration, resulting in more rapid dissipation of residual EO. “It can take a process that may occupy five to 10 days and shorten it to perhaps one day,” Houghtling reports. “This is arguably the biggest advance in the EO sterilization process since its beginning in the 1950s.”